Enhanced TDS
Identification & Functionality
- Chemical Family
- Chemical Name
- Raw Material Origin
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
Applications & Uses
- Markets
- Applications
- Segments
- Applications
- Dosage Form
- Manufacturing Technology
Properties
- Physical Form
- Physico-Chemical Properties
Value Units Test Method / Conditions Loss on Drying max. 6 % G 5-6 Viscosity (1.2% Solids, 120 Second) 39 - 91 cPs A 11a-4 Assay for Carboxymethylcellulose Sodium (Based on NF Equivalency) 8.3 - 13.8 % A 11 – 1 pH 6 - 8 A 11 - 5 Heavy Metals max. 0.001 % G 5-7 Sieve Fraction (60 mesh) max. 0.1 - G 5 - 9a Sieve Fraction (325 mesh) max. 45 wt% G 5 - 9a Residue on Ignition max. 5.0 wt% G 5-11 Identification Pass - A 11 - 2b - Microbiological Values
Value Units Test Method / Conditions Total Aerobic Plate Count max. 1000 cfu/g M 20 - 19 Yeast and Mold Count max. 100 cfu/g M 20 - 20 Pseudomonas aeruginosa None Present /10g M 20 - 21 E. coli None Present /10g M 20 - 22 Staphylococcus aureus None Present /10g M 20 - 23 Salmonella Species None Present /10g M 20 - 24 - Specifications
Value Units Test Method / Conditions NaCMC Content 8.3 - 13.8 % - Viscosity (at 2.6% Solid) 39 - 91 cPs -
Regulatory & Compliance
- Certifications & Compliance Search Terms
- Chemical Inventories
- Quality Standards
Technical Details & Test Data
- Typical Nutrient Information/100g
US EU/Australia/NZ Estimated Caloric Value 18 Cal 189 Cal/ 790 kJoules
Calories from Fat 0 Cal 0 Cal Total Fat 0 g 0 g Saturated Fat 0 g 0 g Trans-Fat 0 g 0 g Polyunsaturated Fat 0 g 0 g Monounsaturated Fat 0 g 0 g Cholesterol 0 mg 0 mg Sodium 940 mg 940 mg Salt -g 2.35 g Potassium 0 mg 0 mg Total Carbohydrate 94 g 0 g Sugars (Total) 0 g 0 g Added Sugars 0 g 0 g Starch 0 g 0 g Total Dietary Fiber 85 g 94 g Dietary Fiber (Soluble) 0 g 9 g Dietary Fiber (Insoluble) 85 g 85 g Protein 0 g 0 g Vitamin A 0 mcg 0 mcg Vitamin C 0 mg 0 mg Vitamin D 0 mcg 0 mcg Calcium 1 mg 1 mg Iron 0.3 mg 0.3 mg Phosphorous 0 mg 0 mg Magnesium 1 mg 1 mg Moisture 4 g 4 g Ash 2 g 2 g Organic acid 0 g 0 g US: Calories = [(Total Carbs + Protein) * 4 Cal/g + (Fat * 9 Cal/g)]. Soluble fiber is included in Total Carbs, but calorie contribution is calculated at 2 Cal/g. In the US, food manufacturers have the option to exclude the amount of insoluble fiber from the calorie calculation.
EU: Calories = ([Carbs (sugars + starch ) + Protein] * 4 Cal/g) + (Fat * 9 Cal/g) + (All dietary fiber * 2 Cal/g) + (Organic acids * 3 Cal/g)). In Europe, fiber is listed separately and is not included in the carb content. Other calorie conversion factors exist, none of which is relevant to this product.- Dispersing and activating Avicel® cMCC for functionality
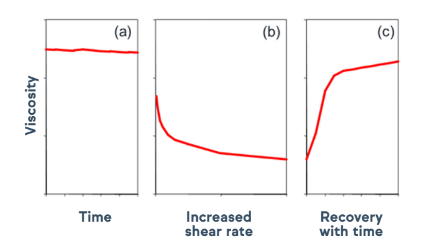
Avicel® cMCC suspending aids demonstrate thixotropic properties, forming gel-like structures at rest and exhibiting shear thinning during shaking and handling. Figure 2 shows the shear thinning that occurs at various shear rates and levels of Avicel® RC-591 in deionized water. Figure 3 shows a schematic to portray the recovery of viscosity and gel-like structure after shaking and handling. This thixotropic behavior profile allows viscous formulations to be thinned for easy swallowing with the shake of a bottle, consistently keeping the desirable mouthfeel until the liquid is poured and swallowed. The gel-like structure recovers with time, ensuring formulation stability and content uniformity during storage.
Activating the gel-like structure is vital to achieving the desired suspension performance. With options for both high- and low-shear dispersion processes, it is no wonder Avicel® cMCC is the industry-leading colloidal MCC for diverse applications.
Avicel® CL-611 can be used where low shear is preferred, enabling the gel-like structure to build and activate in both liquid and reconstitutable suspensions. For liquid formulations, recommended use level starts at ~2.4 percent. For dispersion of dry powders that will be mixed into water, levels of 4 percent or more are recommended to achieve the desired functionality.
Figure 2: Thixotropic characteristics of Avicel® RC-591.
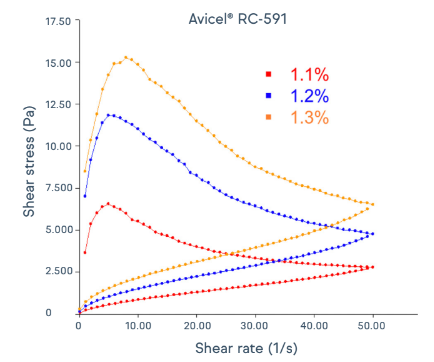
Figure 3: Avicel® RC-591 viscosity consistency at rest (a), during shaking and handling (b), and recovery post handling (c).
Storage & Handling
- Shelf Life
- 3 years
- Shelf Life Information
Three years from date of manufacture shown on product label. This applies to unopened packages, stored under dry and cool conditions.
