Enhanced TDS
Identification & Functionality
- Chemical Family
- Chemical Name
- Raw Material Origin
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
Features & Benefits
- Key Properties
- Biocompatible Ultrapure, for medical use
- Hydrogel formation in presence of divalent cations
- Gelation at constant temperature
- Controllable rheology and gelation
- Long shelf life: 3-5 years depending on product
Applications & Uses
- Markets
- Applications
- Segments
- Applications
- Dosage Form
- Manufacturing Technology
- Product Applications
- Encapsulation of living cells and therapeutic proteins for cell therapy and advanced drug delivery
- Cryoprotection for cell and tissue
- 3D bioprinting ink and polymers
- Scaffolds, foams and cell tissue matrices
- Hydrogels for cell cultures, wound management or advanced drug delivery
- Anti-adhesion and medical device coatings
- Tissue engineering applications such as bone putty binder and matrices for reconstruction
Properties
- Physical Form
- Soluble In
- Typical Properties
Value Units Test Method / Conditions Endotoxins max. 100 EU/g - - Microbiological Values
Value Units Test Method / Conditions Total Viable Count max. 100 cfu/g -
Regulatory & Compliance
- Certifications & Compliance Search Terms
- Quality Standards
Technical Details & Test Data
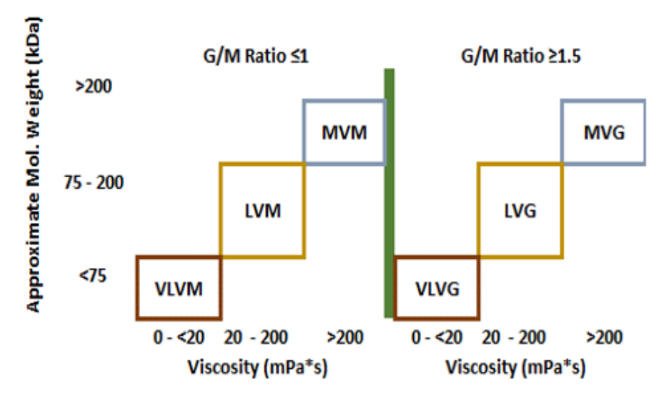
- Viscosity and Molecular Weight
Storage & Handling
- Shelf Life
- 3 years
- Storage Conditions
We recommend refrigerated storage (2-8°C) of all PRONOVA® sodium alginates.
